Can Pyruvate Travel Between Cytoplasm and Mitochondria
Abstract
We have previously demonstrated that the overexpression of enzymes involved in the production of pyruvate, enolase 2 (Eno2) and D-lactate dehydrogenase (Dld3) renders yeast highly sensitive to methylmercury and that the promotion of intracellular pyruvate synthesis may be involved in intensifying the toxicity of methylmercury. In the present study, we showed that the add-on of pyruvate to culture media in non-toxic concentrations significantly enhanced the sensitivity of yeast and human being neuroblastoma cells to methylmercury. The results also suggested that methylmercury promoted the ship of pyruvate into mitochondria and that the increased pyruvate concentrations in mitochondria were involved in intensifying the toxicity of methylmercury without pyruvate being converted to acetyl-CoA. Furthermore, in man neuroblastoma cells, methylmercury treatment alone decreased the mitochondrial membrane potential and the improver of pyruvate led to a farther pregnant subtract. In improver, treatment with North-acetylcysteine (an antioxidant) significantly alleviated the toxicity of methylmercury and significantly inhibited the intensification of methylmercury toxicity past pyruvate. Based on these data, we hypothesize that methylmercury exerts its toxicity by raising the level of pyruvate in mitochondria and that mitochondrial dysfunction and increased levels of reactive oxygen species are involved in the action of pyruvate.
Introduction
Minamata affliction, which is characterized past disorders of the primal nervous organization, is widely known to be caused by ecology methylmercury pollution1,2. Methylmercury becomes highly concentrated in fish at higher levels of the food chain. In contempo years, epidemiological investigations have shown that pregnant women who ingest relatively big amounts of methylmercury (e.yard., through a fish-heavy diet) are at greater risk of giving nascency to children with encephalon developmental disorders3,4. In response, the U.Southward. issued a national warning in 2001 recommending that pregnant women and infants limit their intake of fish, which was followed past similar warnings from Japan, the U.K., Canada, Australia and Norway. Thus, the influence of methylmercury on human health is a worldwide concern. The cellular mechanisms that cause methylmercury toxicity, however, continue to remain unclear, even some 50 years after the outbreak of Minamata affliction.
We found that the ubiquitin-proteasome (Upwardly) system, a protein deposition pathway, plays an of import office as a defence force mechanism against methylmercury toxicityfive,6,7. Previous studies have suggested that some cellular proteins can intensify methylmercury toxicity and that this toxicity is decreased past the Up system, which promotes the deposition of these proteins. Recently, we take successfully identified proteins that are involved in intensifying methylmercury toxicity in yeast; the intracellular levels of these proteins are regulated by the Upwardly system: Dld3, Grs1 and Eno28. Of these three proteins, Dld3 is a D-lactate dehydrogenase involved in the conversion of D-lactose to pyruvate9 and Eno2 (enolase 2) is an enzyme involved in the conversion of glycerate 2-phosphate to phosphoenolpyruvate in the glycolytic pathway10. Nosotros observed that yeast exhibiting overexpression of Dld3 and Eno2, compared to wild-type yeast, were more sensitive to methylmercury, which suggests that an increment in intracellular pyruvate synthesis is somehow involved in intensifying the toxicity of methylmercury. Therefore, the present study sought to examine the part of pyruvate in methylmercury toxicity, using budding yeast as a model organism. Yeast has been established every bit a model organism in which powerful genetic approaches tin be used to elucidate fundamental but complex eukaryotic processes11. Recently, yeast has been used every bit a model organization to report the mechanisms of human neurodegenerative disorders12,13,14. Moreover, the similarities between yeast and man mitochondria facilitate the study of mitochondrial functions15. Examinations were likewise performed using IMR-32 cells, which are homo-derived neuroblastoma cells.
Results
Pyruvate intensifies methylmercury toxicity in yeast
In the glycolytic system, glucose is converted to glycerate 2-phosphate in several stages and then to phosphoenolpyruvate; this conversion is mediated by Eno1 and Eno2. Phosphoenolpyruvate undergoes subsequent conversion to pyruvate, in a procedure mediated by Cdc1916. Because Cdc19 acts downstream of Eno2, we examined how the overexpression of Cdc19 could potentially influence the sensitivity of yeast to methylmercury. In this arroyo, yeast cells that overexpressed Cdc19 demonstrated a loftier sensitivity to methylmercury (Fig. 1A). When non-toxic concentrations of pyruvate were added to the culture media, yeast growth was more intensively inhibited past methylmercury and was dependent on the concentration of pyruvate (Fig. 1B). These results advise that an increase in pyruvate levels in yeast is involved in intensifying the toxicity of methylmercury.
Methylmercury toxicity is intensified past promoting the send of pyruvate into mitochondria
Pyruvate is produced in the cytoplasm and is then transported into the mitochondria, where it is converted to acetyl-CoA; acetyl-CoA subsequently reacts with oxaloacetate to class citrate, thus entering the tricarboxylic acrid (TCA) bicycle. Post-obit the suggestion that pyruvate is involved in methylmercury toxicity, we examined the influence of methylmercury on the level and distribution of pyruvate in yeast. Considering spheroplasting during prison cell fractionation may alter the metabolism or distribution of pyruvate, in this study we spheroplasted yeast cells prior to treatment with methylmercury. Moreover, we used a large quantity of spheroplasts to measure the pyruvate levels because endogenous pyruvate levels tin can be measured by using a large quantity of spheroplasts. The accuracy of cell fractionation was confirmed via Western blotting, using antibodies against cytochrome c oxidase subunit Iii, a mitochondrial protein marking, or iii-phosphoglycerate kinase, a cytoplasmic protein marker (Supplementary Figure one). In this report, pyruvate levels in the cytoplasmic fraction (obtained by removing nuclei from the homogenate) tended to increase after exposure to methylmercury, although the change was not statistically pregnant (Fig. 2A). In add-on, pyruvate levels in the mitochondria exhibited a pregnant increment depending on the concentration of added methylmercury and pyruvate levels in the mail service-mitochondrial fraction (obtained by removing mitochondria from the cytoplasm) in contrast decreased, depending on the concentration of methylmercury (Fig. 2A). In add-on, the cytotoxicity acquired by methylmercury under the present set of weather was 10% or less (data not shown). Measurement of the transport of radioactive pyruvate into isolated mitochondria showed increased transportation of pyruvate into the mitochondria and was dependent on the concentration of methylmercury used during the treatment (Fig. 2B). Based on these observations, we hypothesize that methylmercury promotes the transport of pyruvate into mitochondria.
Pyruvate synthesized in the cytoplasm of yeast is transported into the mitochondrial matrix, a process mediated by the Yil006w transporter that is present in the inner mitochondrial membrane17. Nosotros therefore examined the sensitivity of Yil006w-deleted yeast to methylmercury to determine the relationship betwixt methylmercury toxicity and pyruvate transport into mitochondria. In this approach, Yil006w-deleted yeast (yil006wΔ) demonstrated an enhanced resistance to methylmercury compared to that of wild-type yeast (Fig. 3A). In add-on, in Yil006w-deleted yeast the toxicity of methylmercury was but minimally intensified by pyruvate (Fig. 3A) and pyruvate levels in the mitochondria were lower and were not increased after treatment with methylmercury (Fig. 3B), in contrast to wild-type yeast. Based on these observations, we hypothesize that methylmercury toxicity is intensified by promoting the ship of pyruvate into mitochondria, a process mediated by Yil006w.
Pyruvate is involved in intensifying methylmercury toxicity without being metabolized to acetyl-CoA
Pyruvate that is transported into mitochondria is converted to acetyl-CoA before inbound the TCA wheel and is thus involved in ATP production. Pyruvate dehydrogenase is an enzyme that converts pyruvate to acetyl-CoA and is composed of several proteins: Pda1, Pdb1, Lat1, Lpd1 and Pdx118. To make up one's mind the relationship between methylmercury toxicity and the conversion of pyruvate to acetyl-CoA, nosotros examined the influence of the removal of pyruvate dehydrogenase components Pda1, Pdb1, Lat1 and Lpd1. In this approach, yeast with deletions of the respective components, compared to wild-blazon yeast, consistently exhibited a higher sensitivity to methylmercury (data not shown). We subsequently examined how the add-on of pyruvate to the civilisation media influenced methylmercury toxicity using yeast cells with Lat1 deleted (dihydrolipoyl transacetylase, the active centre of pyruvate dehydrogenase)nineteen,20. In this arroyo, Lat1-deleted yeast (lat1Δ) which are almost completely lack pyruvate dehydrogenase activity were more sensitive to methylmercury than were wild-type yeast and were more sensitive to methylmercury toxicity by pyruvate (Fig. 4A). Like results were obtained with Lpd1-deleted yeast (lpd1Δ) (Fig. 4B). Based on these results, we hypothesize that pyruvate that is transported into mitochondria is involved in intensifying methylmercury toxicity without existence converted to acetyl-CoA.
Methylmercury causes mitochondrial dysfunction by promoting the send of pyruvate into mitochondria in homo neuroblastoma cells
Our observations in yeast suggested that methylmercury produces cytotoxicity past increasing the pyruvate levels in mitochondria. We next investigated the influence of pyruvate on methylmercury toxicity; nosotros used man neuroblastoma cells (IMR-32) because methylmercury is a neurotoxic substance. In this approach, using an Alamar blue assay (Fig. 5A) and an MTT assay (Supplementary Effigy 2), nosotros observed that the addition of non-toxic concentrations of pyruvate to the civilization media significantly enhanced the sensitivity of the cells to methylmercury, a issue like to the response observed in yeast. We next examined the influence of methylmercury on pyruvate levels in the mitochondria of IMR-32 cells. However, information technology is very hard to measure out the levels of endogenous pyruvate in human being cultured cells. Therefore, IMR-32 cells were treated with radioactive pyruvate for 90 min, followed by handling with methylmercury for up to 15 min, before the level of radioactive pyruvate in mitochondrial fractions was measured with a liquid scintillator. In this arroyo, a cursory treatment with methylmercury significantly increased the level of radioactive pyruvate in the mitochondria (Fig. 5B). In add-on, the effectiveness of cell fractionation was confirmed via western blotting, using antibodies confronting cytochrome c oxidase subunit IV, a mitochondrial mark poly peptide, or glyceraldehyde 3-phosphate dehydrogenase, a cytoplasmic poly peptide marker (Supplementary Figure 3). These results suggested that methylmercury causes cellular disorders past promoting the ship of pyruvate into mitochondria in human-derived IMR-32 cells as well every bit in yeast.
Mitochondria are organelles that produce free energy by promoting membrane electrogenesis, due to a pH or potential difference between the outer side and matrix side of the inner membrane. If the membrane potential decreases, depression-molecular-weight substances (including protons) flow into the mitochondria, causing mitochondrial dysfunction21,22. The results shown in Fig. four suggested that pyruvate that is transported into mitochondria is involved in intensifying methylmercury toxicity without being converted to acetyl-CoA. There is a possibility that pyruvate transported into mitochondria influences the mitochondrial membrane potential as an organic acid. We therefore investigated the relationship betwixt the mitochondrial membrane potential and the intensification of methylmercury toxicity mediated past pyruvate. The membrane potential was measured using rhodamine 123, a cationic fluorescent substance that accumulates in response to an increase of anions on the matrix side of the mitochondria23. In this approach, treatment with methylmercury alone for iii hr decreased the mitochondrial membrane potential and the addition of pyruvate resulted in an additional, significant decrease (Fig. 5C). This result suggests that mitochondrial dysfunction is involved in the intensification of methylmercury toxicity mediated past pyruvate. In addition, methylmercury is known to promote the production of reactive oxygen species in mitochondria24,25,26,27,28. We therefore investigated the human relationship between reactive oxygen species and the intensification of methylmercury toxicity mediated by pyruvate. In this approach, treatment with methylmercury alone for half dozen 60 minutes increased the intracellular levels of reactive oxygen species and the addition of pyruvate further increased the product of reactive oxygen species, depending on the concentration of pyruvate used (Fig. 5D). However, treatment with pyruvate alone did not influence the intracellular levels of reactive oxygen species. In addition, the levels of reactive oxygen species were non inverse after treatment for iii hr with methylmercury solitary or in combination with pyruvate (data not shown). These results propose that the production of reactive oxygen species via the reduction of the mitochondrial membrane potential may be involved in the intensification of methylmercury toxicity past pyruvate. Next, we examined the influence of N-acetylcysteine (NAC), an antioxidant, on the intensification of methylmercury toxicity mediated by pyruvate. NAC is a cysteine derivative that may demark to methylmercury in culture media. Therefore, nosotros treated IMR-32 cells with NAC for half-dozen hr and subsequently replaced the civilization media to remove NAC prior to treatment with methylmercury or pyruvate. In this arroyo, treatment with NAC significantly alleviated methylmercury toxicity and significantly inhibited the intensification of methylmercury toxicity mediated by pyruvate (Fig. 5F). In addition, the cell viability was not changed after treatment with methylmercury alone or in combination with pyruvate for 6 hour (information not shown). These results strongly suggest that reactive oxygen species are involved in the intensification of methylmercury toxicity mediated by pyruvate.
Give-and-take
Pyruvate produced from glycolysis is involved in the product of ATP and in homeostasis of carbohydrates, fats and amino acids29,xxx. It has likewise been reported that some cells release pyruvate into claret plasma and serum and the released pyruvate reacts with extracellular H2O2 independently of enzymes to produce an antioxidative action31,32. Interestingly, our study found that excessive pyruvate transported into mitochondria intensified the toxicity of methylmercury without being metabolized to acetyl-CoA.
Excessive pyruvate added to culture media did not change pyruvate levels in mitochondria and did not pb to cytotoxicity (information not shown), indicating that the normal level of pyruvate in mitochondria is under strict control. However, in the presence of methylmercury, nosotros observed enhanced send of pyruvate into mitochondria. Furthermore, the deletion of the Yil006w transporter (which is involved in transporting pyruvate into mitochondria) did not result in any intensification of pyruvate-mediated methylmercury toxicity. Based on these observations, we can hypothesize that the toxicity of methylmercury is intensified past increasing the pyruvate levels in mitochondria. Normally, pyruvate is involved in the production of ATP via the TCA cycle, following its transportation into mitochondria. However, deletion of pyruvate dehydrogenase, which catalyses the metabolism of pyruvate, resulted in a further intensification of pyruvate-mediated methylmercury toxicity, suggesting that pyruvate intensifies the toxicity of methylmercury without being metabolized in the TCA bike.
However, it cannot exist excluded that pyruvate dehydrogenase is somehow involved in the pyruvate-mediated intensification of methylmercury toxicity. Pyruvate dehydrogenase, which converts pyruvate to acetyl-CoA, is a complex composed of three enzymes and the chief active site is comprised of lipoic acid bound to a Lys residue of dihydrolipoyl transacetylase (Lat1)33. This lipoic acid has sulfhydryl groups that form a disulphide bond in a reversible manner and the resulting redox reaction decarboxylates pyruvate and transfers an acetyl group to the decarboxylated pyruvate. Methylmercury is known to demark strongly to sulfhydryl groups and block the activeness of sulfhydryl enzymes34. This leaves the possibility that methylmercury transported into mitochondria binds to the sulfhydryl groups of Lat1 and thus blocks the action of pyruvate dehydrogenase, resulting in aggregating of pyruvate within the mitochondria. Therefore, we examined the influence of methylmercury on the activeness of pyruvate dehydrogenase. In this approach, treatment with methylmercury caused only an ~10% decrease in the activeness of pyruvate dehydrogenase, a small difference compared to the control in yeast and IMR-32 cells (data non shown). We can thus hypothesize that blockage of pyruvate dehydrogenase action by methylmercury is essentially not involved when methylmercury increases the pyruvate levels in mitochondria.
Some authors, in examining mitochondria isolated from rat liver cells, have suggested that methylmercury opens a mitochondrial membrane permeability transition pore and promotes influx of anions such as nitrate, thus inducing mitochondrial swelling and leading to cell expiry35. Previous studies accept indicated that pyruvate strengthens the dihydrolipoate-induced mitochondrial permeability transition and mitochondrial swelling in isolated rat liver mitochondria36. The increment in mitochondrial permeability has been reported to exist involved in neuronal injury37 and mitochondrial swelling has been found to exist induced in the cortices in an Alzheimer disease mouse model38. Therefore, the disruption of the mitochondrial permeability transition and of mitochondrial swelling may exist involved in the mechanism of pyruvate-mediated methylmercury toxicity. Information technology is possible that methylmercury has some influence on transporters that are present in mitochondria, such every bit Yil006w and hence promotes the transport of pyruvate into mitochondria. The results in Fig. 3B evidence that pyruvate levels in mitochondria from Yil006-deleted yeast were approximately thirty% of those in wild-type yeast, indicating that mitochondria also have some other mechanism involved in the transport of pyruvate in addition to Yil006w. However, transport mechanisms other than Yil006w do non appear to be involved when methylmercury promotes the ship of pyruvate into mitochondria, given that methylmercury did not increase pyruvate concentrations in mitochondria following the deletion of Yil006w.
One study has reported that methylmercury exhibits cytotoxicity by increasing mitochondrial membrane permeability, thus increasing the release of calcium from mitochondria to the cytoplasm39,40. Other reports have raised the possibility that methylmercury promotes the production of reactive oxygen species by inhibiting the activeness of complex III of the mitochondrial electron ship chain41 and that methylmercury blocks production of ATP by inhibiting the role of complex 442. Thus, mitochondria can exist regarded as intracellular targets for methylmercury. The addition of pyruvate minimally influenced the cytotoxicity of cadmium, arsenic trioxide and other substances (data not shown), which suggests that the intensification of toxicity mediated by pyruvate may be specific to methylmercury toxicity.
Our present findings suggest the existence of a new mechanism for the toxicity of methylmercury that targets mitochondria, in addition to the to a higher place-mentioned known toxic mechanism of methylmercury acting through mitochondria. The mechanisms related to methylmercury toxicity may exist clarified by studying in detail the human relationship betwixt the level of pyruvate in mitochondria and the known toxic machinery of methylmercury acting via mitochondria.
Materials and Methods
Yeast strain and growth culture conditions
Saccharomyces cerevisiae BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and deletion (knock-out) strains constructed past insertion of kanMX4 cassettes (conferring G418 resistance equally a selective marker of the BY4742 genome) were obtained from Euroscarf (Frankfurt, Frg). Yeast cells were grown in yeast extract-peptone-dextrose (YPD) medium or synthetic dextrose (SD) medium at 30 °C. Plasmid Deoxyribonucleic acid was introduced into BY4742 cells using the high-efficiency lithium acetate transformation method43.
Yeast growth curve
Yeast cells (6.25 × 105 cells/mL) were grown in synthetic dextrose (SD) medium containing methylmercuric chloride and sodium pyruvate at the indicated concentrations in 96-well plates. The yeast cells were incubated for 48 60 minutes at 30 °C with shaking. The absorbance of each culture at 600 nm was measured every 3 hour to quantify jail cell growth.
Spheroplasting and fractionation
Spheroplasts and mitochondria were prepared co-ordinate to previously published methods44. Yeast cells were grown in 1 Fifty of SD liquid medium until the optical density at 600 nm was approximately 1.7 (xxi cells/L). The yeast cells were collected and incubated in 60 mL of Tris buffer (0.i M Tris-Theniv, pH nine.iv, ten mM DTT) for x min at 30 °C with gentle shaking (100 rpm, Bio-shaker BR-40LF, Taitec, Saitama, Japan). Later the yeast cells were centrifuged at 2,000 × thou for v min, the pellet was washed with 60 mL of spheroplasting buffer (1.2 One thousand sorbitol, twenty mM KPi, pH 7.4), nerveless by centrifugation (2,000 × g for 5 min) and and so suspended in sixty mL of spheroplasting buffer. For digesting yeast jail cell walls, the suspension was incubated with zymolyase (2 mg zymolyase/g yeast) for 15 to xxx min at 30 °C with gentle shaking (100 rpm, Bio-shaker BR-40LF, Taitec). To ostend spheroplasting, a pocket-size aliquot (20 μL) of yeast intermission was added to 1 mL of distilled h2o. When the yeast cells appeared to be collapsed due to the osmotic force per unit area, they were considered to be completely spheroplasted.
The spheroplasts (ii × xten cells) were suspended in 200 mL of SD medium containing 1.2 Thousand sorbitol to an optical density of 0.85 at 600 nm and treated with methylmercuric chloride for 1 60 minutes at 30 °C with gentle shaking (100 rpm, Bio-shaker BR-40LF, Taitec). Methylmercury-treated spheroplasts were washed twice with ice-cold spheroplasting buffer, suspended in 3 mL of mitochondrial isolation buffer (MIB) (0.six Grand sorbitol, 20 mM HEPES-KOH, pH 7.4, i mM PMSF, 0.5 mM EDTA) so homogenized on ice with a Dounce homogenizer (Wheaton, Millville, NJ, U.s.a.) and 20 strokes with a tight-fitting pestle. The homogenate was divided into mitochondrial, post-mitochondrial and post-nuclear fractions.
For the mitochondrial fraction, part of the homogenate was centrifuged at ane,000 × g for 5 min. The supernatant was transferred to another tube and the pellet was resuspended in 2 mL of MIB followed by homogenization and centrifugation at 1,500 × g for 5 min. The supernatant was combined with the previously prepared supernatant. The supernatant was centrifuged at 12,000 × g for ten min and the pellet was suspended in 10 mL of MIB. After centrifugation at 1,500 × g for 5 min, the supernatant was collected and centrifuged at 12,000 × thousand for 10 min and the pellet was suspended in MIB without phenylmethylsulfonyl fluoride. The suspension was centrifuged at 12,000 × g for x min and the pellet (the mitochondrial fraction) was suspended in 1 mL of PBS.
For the mail service-mitochondrial fraction, the homogenate was centrifuged at 20,000 × 1000 for 30 min and this supernatant was used every bit the post-mitochondrial fraction. For the post-nuclear fraction, the homogenate was centrifuged at ane,500 × g for 30 min. This supernatant was used as the post-nuclear fraction.
Quantification of pyruvate
The poly peptide concentration from each fraction was adamant using a DC protein analysis (Bio-Rad, Hercules, CA, United states of america) and a one mL fraction was used for the quantification of pyruvate. Each fraction was lysed by sonication for 30 sec (output 4, duty xxx%, Branson Sonifier 450). The lysate was filtered via centrifugation (8,000 × one thousand) for x min using a 0.1 μm pore micro-filter. The filtrate was incubated with 500 μM β-NADH and 2 units of Fifty-lactate dehydrogenase in 0.5 M Tris buffer, pH seven.4, at 37 °C for 30 sec. The consumption of β-NADH was determined using the absorbance at 340 nm to represent the amount of pyruvate45.
IMR-32 cell culture and viability
Human neuroblastoma (IMR-32 cells) were cultured in Dulbecco'due south Modified Eagle's Medium (DMEM) (Sigma, St. Louis, MO, U.s.) supplemented with 10% (v/5) foetal bovine serum (Bio-west, Kansas City, MO, USA), 0.06% L-glutamine and 100 U/mL penicillin (Invitrogen, G Island, NY, U.s.) at 37 °C in a humidified incubator containing 5% (v/v) CO2. IMR-32 cells (2 × 10iv cells/well) were seeded into 96-well plates for 24 hr and treated with methylmercuric chloride and/or pyruvate at the indicated concentrations for 24 hour. Jail cell viability was measured using a ten% Alamar blue solution using an excitation wavelength of 544 nm and an emission wavelength of 590 nm.
Crude mitochondrial isolation from IMR-32 cells
Rough mitochondria were isolated according to previously published methods46. The cells were suspended in mitochondrial isolation buffer (10 mM NaCl, 1.5 mM CaCl2, x mM Tris-HCl, pH 7.5) and incubated on ice for 10 min. After the incubation, the jail cell break was homogenized using 30 strokes with a Dounce homogenizer with a tight-fitting pestle on ice. The homogenate was centrifuged at 600 × g for 10 min, followed by centrifugation of the supernatant at half dozen,000 × yard for 10 min. The pellet was washed twice with mitochondrial isolation buffer then suspended in PBS and used as the rough mitochondrial fraction.
Pyruvate incorporation into mitochondria in IMR-32 cells
IMR-32 cells (6 × 106 cells) were seeded into xv cm culture dishes in 30 mL pyruvate-free DMEM and cultured for 24 hour. The cells were treated with 0.05 μCi/mL of [two-14C] pyruvate (PerkinElmer Inc., Waltham, MA, U.s.a.) for 90 min followed past addition of methylmercuric chloride to the prison cell culture. Later on incubation, the mitochondria were isolated from the cells. The amount of [2-14C] pyruvate incorporated into the mitochondria was measured using a liquid scintillation counter and was normalized to the amount of mitochondrial protein47.
Mitochondrial membrane potential
IMR-32 cells were seeded into 6-well plates at a concentration of four × 10five cells/2 mL/well. Subsequently a 24 hr cultivation period, the indicated concentrations of methylmercuric chloride and/or pyruvate were added, followed by incubation for 3 hour. Later incubation and washing with Hank'south Balanced Salt Solution (HBSS) buffer, the cells were incubated with 5 μM rhodamine 123 (Sigma) in HBSS buffer for 30 min in the dark. Rhodamine 123-treated cells were collected with 600 μL of water ice-common cold HBSS buffer. To measure out the intensity of rhodamine 123, 100 μL of jail cell break was transferred to one-half of the wells of a 96-well plate and the fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Cells in 300 μL suspensions were counted for the normalization of the mitochondrial membrane potential48,49.
Boosted Information
How to cite this article: Lee, J.-Y. et al. Transport of pyruvate into mitochondria is involved in methylmercury toxicity. Sci. Rep. 6, 21528; doi: 10.1038/srep21528 (2016).
References
-
Castoldi, A. F., Coccini, T. & Manzo, L. Neurotoxic and molecular effects of methylmercury in humans. Rev. Environ. Health 18, 19–31 (2003).
-
Sanfeliu, C., Sebastia, J., Cristofol, R. & Rodriguez-Farre, E. Neurotoxicity of organomercurial compounds. Neurotox. Res. 5, 283–305 (2003).
-
Grandjean, P. et al. Impact of maternal seafood diet on fetal exposure to mercury, selenium and lead. Arch. Environ. Wellness 47, 185–195, 10.1080/00039896.1992.9938348 (1992).
-
Grandjean, P. et al. Cognitive arrears in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 19, 417–428 (1997).
-
Hwang, One thousand. Westward., Furuchi, T. & Naganuma, A. A ubiquitin-proteasome system is responsible for the protection of yeast and human cells against methylmercury. FASEB J. sixteen, 709–711, ten.1096/fj.01-0899fje (2002).
-
Hwang, 1000. W., Ishida, Y. & Naganuma, A. Identification of F-box proteins that are involved in resistance to methylmercury in Saccharomyces cerevisiae. FEBS Lett. 580, 6813–6818, x.1016/j.febslet.2006.11.045 (2006).
-
Hwang, Thou. W., Mastuyama, F., Takahashi, T., Lee, J. Y. & Naganuma, A. Deletion of the ubiquitin-conjugating enzyme Ubc2 confers resistance to methylmercury in budding yeast by promoting Whi2 degradation. J. Toxicol. Sci. 38, 301–303 (2013).
-
Lee, J. Y., Ishida, Y., Kuge, S., Naganuma, A. & Hwang, K. Due west. Identification of substrates of F-box protein involved in methylmercury toxicity in yeast cells. FEBS Lett. 589, 2720–2725, ten.1016/j.febslet.2015.08.016 (2015).
-
Chelstowska, A., Liu, Z., Jia, Y., Amberg, D. & Butow, R. A. Signalling betwixt mitochondria and the nucleus regulates the expression of a new D-lactate dehydrogenase activity in yeast. Yeast 15, 1377–1391, x.1002/(SICI)1097-0061(19990930)15:13<1377::AID-YEA473>iii.0.CO;2-0 (1999).
-
McAlister, L. & Kingdom of the netherlands, Grand. J. Targeted deletion of a yeast enolase structural factor. Identification and isolation of yeast enolase isozymes. J. Biol. Chem. 257, 7181–7188 (1982).
-
Smith, M. G. & Snyder, M. Yeast as a model for human disease. Curr. Protoc. Hum. Genet. Chapter 15, Unit of measurement xv 16, ten.1002/0471142905.hg1506s48 (2006).
-
Franssens, V. et al. The benefits of humanized yeast models to written report Parkinson'southward disease. Oxid. Med. Cell. Longev. 2013, 760629, 10.1155/2013/760629 (2013).
-
Ocampo, A. & Barrientos, A. From the bakery to the brain business organization: developing inducible yeast models of human neurodegenerative disorders. Biotechniques 45, 7–xiv, 10.2144/000112746 (2008).
-
Tenreiro, S., Munder, M. C., Alberti, S. & Outeiro, T. F. Harnessing the ability of yeast to unravel the molecular ground of neurodegeneration. J. Neurochem. 127, 438–452, 10.1111/jnc.12271 (2013).
-
Lasserre, J. P. et al. Yeast equally a arrangement for modeling mitochondrial affliction mechanisms and discovering therapies. Dis. Model. Mech. 8, 509–526, 10.1242/dmm.020438 (2015).
-
Fraenkel, D. G. The pinnacle genes: on the distance from transcript to office in yeast glycolysis. Curr. Opin. Microbiol. 6, 198–201 (2003).
-
Hildyard, J. C. & Halestrap, A. P. Identification of the mitochondrial pyruvate carrier in Saccharomyces cerevisiae. Biochem. J. 374, 607–611, x.1042/BJ20030995 (2003).
-
Steensma, H. Y., Holterman, 50., Dekker, I., van Sluis, C. A. & Wenzel, T. J. Molecular cloning of the gene for the E1 alpha subunit of the pyruvate dehydrogenase complex from Saccharomyces cerevisiae. Eur. J. Biochem. 191, 769–774 (1990).
-
Behal, R. H., Buxton, D. B., Robertson, J. M. & Olson, M. S. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu. Rev. Nutr. 13, 497–520, 10.1146/annurev.nu.13.070193.002433 (1993).
-
Pronk, J. T., Yde Steensma, H. & Van Dijken, J. P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12, 1607–1633, 10.1002/(SICI)1097-0061(199612)12:16<1607::Help-YEA70>3.0.CO;2–4 (1996).
-
Daiber, A. Redox signaling (cantankerous-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 1797, 897–906, ten.1016/j.bbabio.2010.01.032 (2010).
-
Nicholls, D. K. Mitochondrial ion circuits. Essays Biochem. 47, 25–35, 10.1042/bse0470025 (2010).
-
Kahlert, S., Zundorf, G. & Reiser, G. Detection of de- and hyperpolarization of mitochondria of cultured astrocytes and neurons past the cationic fluorescent dye rhodamine 123. J. Neurosci. Methods 171, 87–92, 10.1016/j.jneumeth.2008.02.015 (2008).
-
Ali, South. F., LeBel, C. P. & Bondy, S. C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology thirteen, 637–648 (1992).
-
Mundy, W. R. & Freudenrich, T. Thou. Sensitivity of immature neurons in civilization to metal-induced changes in reactive oxygen species and intracellular free calcium. Neurotoxicology 21, 1135–1144 (2000).
-
Naganuma, A. et al. Overexpression of manganese-superoxide dismutase prevents methylmercury toxicity in HeLa cells. Life Sci. 62, PL157–161 (1998).
-
Shanker, G., Syversen, T. & Aschner, 1000. Astrocyte-mediated methylmercury neurotoxicity. Biol. Trace. Elem. Res. 95, i–10, 10.1385/BTER:95:1:1 (2003).
-
Usuki, F., Fujita, E. & Sasagawa, N. Methylmercury activates ASK1/JNK signaling pathways, leading to apoptosis due to both mitochondria- and endoplasmic reticulum (ER)-generated processes in myogenic cell lines. Neurotoxicology 29, 22–30, 10.1016/j.neuro.2007.08.011 (2008).
-
Camarasa, C., Grivet, J. P. & Dequin, S. Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology 149, 2669–2678 (2003).
-
Jeoung, N. H., Harris, C. R. & Harris, R. A. Regulation of pyruvate metabolism in metabolic-related diseases. Rev. Endocr. Metab. Disord. 15, 99–110, x.1007/s11154-013-9284-ii (2014).
-
Das, U. N. Pyruvate is an endogenous anti-inflammatory and anti-oxidant molecule. Med. Sci. Monit. 12, RA79–84 (2006).
-
Long, L. H. & Halliwell, B. Artefacts in jail cell civilization: pyruvate equally a scavenger of hydrogen peroxide generated past ascorbate or epigallocatechin gallate in prison cell culture media. Biochem. Biophys. Res. Commun. 388, 700–704, 10.1016/j.bbrc.2009.08.069 (2009).
-
Reed, L. J. & Hackert, M. L. Structure-function relationships in dihydrolipoamide acyltransferases. J. Biol. Chem. 265, 8971–8974 (1990).
-
Franco, J. L. et al. Cerebellar thiol status and motor deficit subsequently lactational exposure to methylmercury. Environ. Res. 102, 22–28, 10.1016/j.envres.2006.02.003 (2006).
-
Bragadin, 1000., Marton, D., Manente, S., Grasso, G. & Toninello, A. Methylmercury induces the opening of the permeability transition pore in rat liver mitochondria. J. Inorg. Biochem. 89, 159–162 (2002).
-
Morkunaite, S., Teplova, V. 5. & Saris, N. East. Mechanism of dihydrolipoate stimulation of the mitochondrial permeability transition: effect of unlike respiratory substrates. IUBMB Life 49, 211–216, 10.1080/713803622 (2000).
-
Galluzzi, L., Blomgren, One thousand. & Kroemer, G. Mitochondrial membrane permeabilization in neuronal injury. Nat. Rev. Neurosci. x, 481–494, 10.1038/nrn2665 (2009).
-
Du, H., Guo, L., Zhang, West., Rydzewska, Thousand. & Yan, S. Cyclophilin D deficiency improves mitochondrial role and learning/retentivity in aging Alzheimer illness mouse model. Neurobiol. Aging 32, 398–406, ten.1016/j.neurobiolaging.2009.03.003 (2011).
-
Chang, J. Y. & Tsai, P. F. Prevention of methylmercury-induced mitochondrial depolarization, glutathione depletion and prison cell death by 15-deoxy-delta-12,xiv-prostaglandin J(ii). Neurotoxicology 29, 1054–1061, 10.1016/j.neuro.2008.08.003 (2008).
-
Lee, J. Y., Hwang, Thousand. W. & Naganuma, A. Rip1 enhances methylmercury toxicity through production of reactive oxygen species (ROS) in budding yeast. J. Toxicol. Sci. 34, 715–717 (2009).
-
Drose, Southward. & Brandt, U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 283, 21649–21654, ten.1074/jbc.M803236200 (2008).
-
Chang, C. M., Yu, C. C., Lu, H. T., Chou, Y. F. & Huang, R. F. Folate deprivation promotes mitochondrial oxidative decay: Deoxyribonucleic acid large deletions, cytochrome c oxidase dysfunction, membrane depolarization and superoxide overproduction in rat liver. Br. J. Nutr. 97, 855–863, x.1017/S0007114507666410 (2007).
-
Hwang, G. W., Murai, Y., Takahashi, T. & Naganuma, A. The protein transportation pathway from Golgi to vacuoles via endosomes plays a function in enhancement of methylmercury toxicity. Sci. Rep. iv, 5888, 10.1038/srep05888 (2014).
-
Daum, G., Bohni, P. C. & Schatz, G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257, 13028–13033 (1982).
-
de Marcos, S., Galban, J., Alonsa, C. & Castillo, J. R. Intrinsic molecular fluorescence of lactate dehydrogenase: an analytical culling for enzymic decision of pyruvate. Analyst. 122, 355–359 (1997).
-
Kang, B. H. et al. Regulation of tumor prison cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell 131, 257–270, 10.1016/j.cell.2007.08.028 (2007).
-
Pande, S. V. & Parvin, R. Pyruvate and acetoacetate transport in mitochondria. A reappraisal. J. Biol. Chem. 253, 1565–1573 (1978).
-
Allen, J. D., Jackson, Due south. C. & Schinkel, A. H. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Cancer Res. 62, 2294–2299 (2002).
-
Hong, Grand. Y. et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis 23, 1919–1925 (2002).
Acknowledgements
This piece of work was supported by a Grant-in-Assist for Scientific Research (KAKENHI 24249008 and 25281023) by the Nippon Society for the Promotion of Science.
Author information
Affiliations
Contributions
G.Westward.H. and A.N. designed the experiments and wrote the manuscript. Thousand.Due west.H. prepared Figure 1. J.Y.L. and Y.I. prepared Figures 2, iii, 4. J.Y.Fifty. and T.T. prepared Effigy v. G.W.H. and A.Northward. reviewed the manuscript.
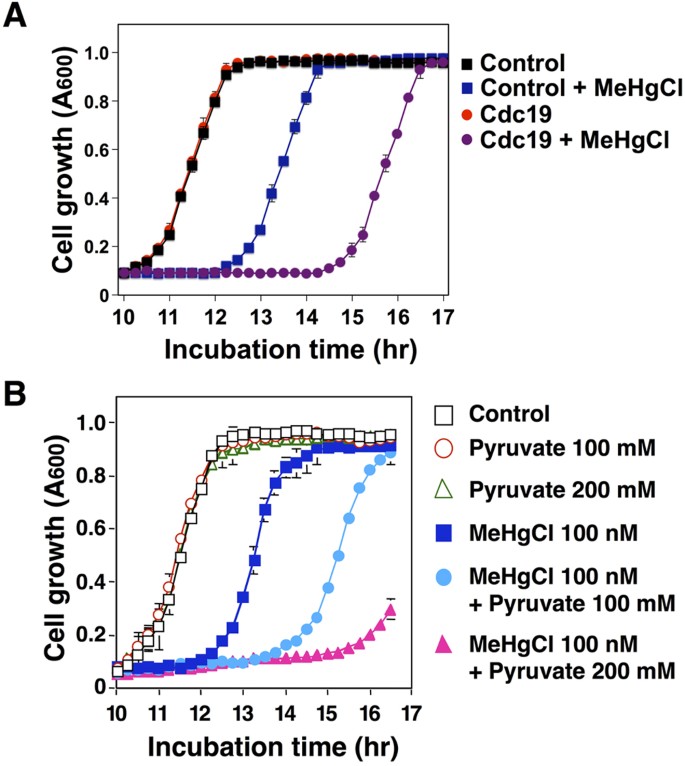
Pyruvate intensifies methylmercury toxicity in yeast.
(A). Yeast cells (six.25 × 105 cells/mL) expressing the indicated plasmids were cultured at thirty °C in SD liquid media containing methylmercuric chloride (MeHgCl; 100 nM). The absorbance was measured spectrophotometrically at 600 nm every 15 min for 24 hr. The information correspond the mean ± S.D. of iii cultures. The absence of a bracket indicates that the S.D. was within the area of the symbol. (B). Wild-type yeast cells (six.25 × 105 cells/mL) were cultured at 30 °C in SD liquid medium containing methylmercuric chloride and/or sodium pyruvate at the indicated concentrations. The absorbance was measured spectrophotometrically at 600 nm every 15 min for 24 hour. The information represent the mean ± S.D. of three cultures. The absence of a bracket indicates that the S.D. was inside the area of the symbol.
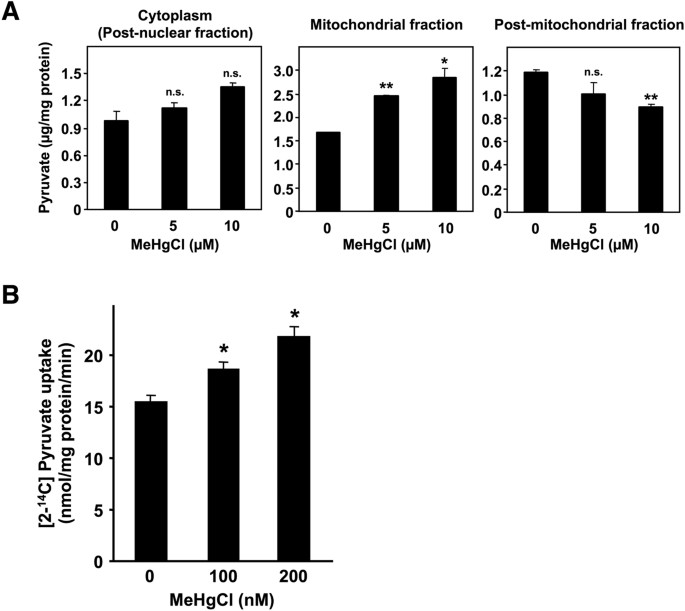
Methylmercury induces the send of pyruvate into the mitochondria in yeast.
(A). Spheroplasts (108 cells/mL) were incubated with gentle shaking at 30 °C in sorbitol-containing SD liquid medium that also contained methylmercuric chloride at the indicated concentrations. Later a 1 hr incubation, post-nuclear, mitochondrial and mail-mitochondrial fractions were prepared at 4 °C. The pyruvate levels of each fraction were measured by β-NADH consumption using pyruvate reduction at 340 nm. Significant differences were observed relative to the control grouping without methylmercury treatment. n.southward.: no significance, *p < 0.05, **p < 0.01 (B). The uptake of 14C-labelled pyruvate into intact mitochondria was measured after handling with methylmercuric acid in vitro. The imported pyruvate was quantified using a liquid scintillation counter and normalized to the amount of mitochondrial poly peptide. Significant differences were observed relative to the command group (without methylmercury treatment). *p < 0.01.
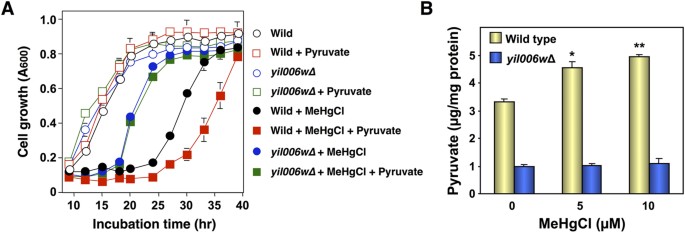
Yil006w is involved in the methylmercury-mediated ship of pyruvate into mitochondria in yeast.
(A). Yeast cells were cultured at 30 °C in SD liquid medium containing methylmercuric chloride and/or sodium pyruvate at the indicated concentrations. The absorbance at 600 nm was measured using a spectrophotometer every 3 60 minutes for 48 hr. The data represent the mean ± S.D. of iii cultures. The absence of a bracket indicates that the Due south.D. was within the area of the symbol. (B). Spheroplasts were incubated with gentle shaking at xxx °C in sorbitol-SD medium containing methylmercuric chloride at the indicated concentrations. After a i hr incubation, the mitochondrial fraction was prepared at four °C. Pyruvate levels were measured by β-NADH consumption using pyruvate reduction at 34 nm. Significant differences were observed compared to the control group without methylmercury treatment. *p < 0.05, **p < 0.01.
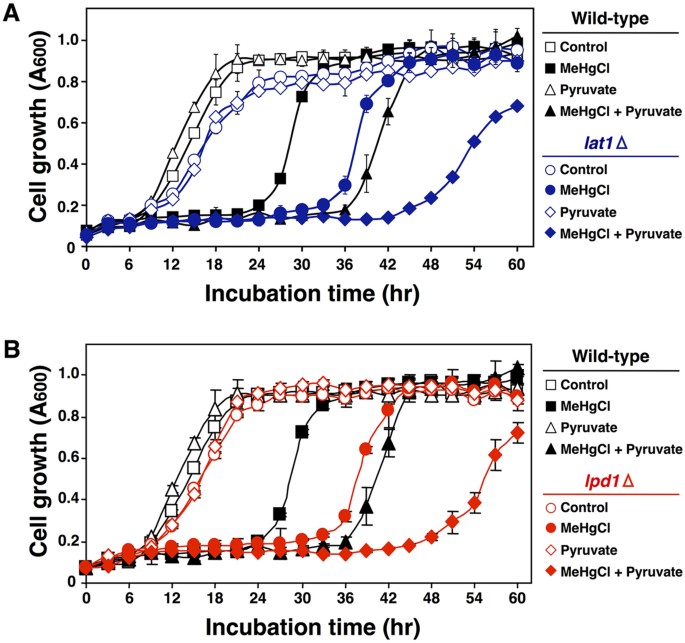
Pyruvate is not converted to acetyl-CoA in the mitochondria matrix and is involved in methylmercury toxicity.
Yeast cells with deletions of the indicated gene(s) ((A) LAT1, (B) LPD1) were cultured at xxx °C in SD liquid medium containing methylmercuric chloride and/or sodium pyruvate at the indicated concentrations. The absorbance was measured with a spectrophotometer at 600 nm every 3 hr for 48 hour. The data represent the mean ± S.D. of three cultures. The absence of a subclass indicates that the S.D. was within the area of the symbol.
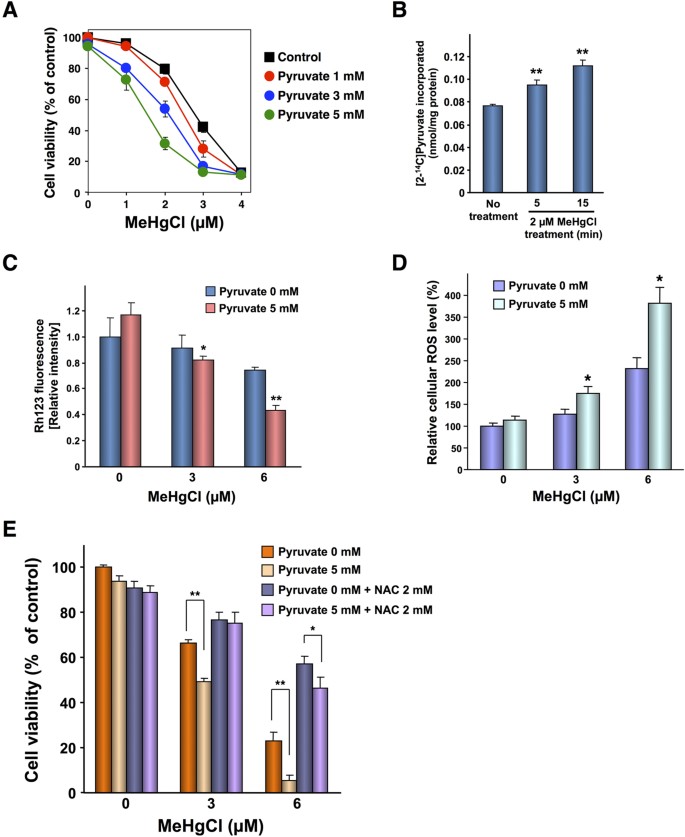
Methylmercury causes mitochondrial dysfunction by promoting the uptake of pyruvate into the mitochondria in IMR-32 cells.
(A). Prison cell viability was measured using an Alamar blue solution. The data represent the mean ± S.D. of three cultures. The absence of a bracket indicates that the S.D. was within the expanse of the symbol. (B). [ii-14C] Pyruvate levels in mitochondria were measured using a liquid scintillation counter. Significant differences were observed compared to the control grouping without pyruvate treatment. *p < 0.05, **p < 0.01 (C). Rhodamine 123 levels were measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Significant differences were observed relative to the control group without pyruvate treatment. *p < 0.05, **p < 0.01 (D). IMR-32 cells (2 × x4 cells/80 μL) were seeded into each well of a 96-well plate. After incubation for 24 60 minutes, 10 μM H2DCF-DA was added to each well. I 60 minutes later, the cells were treated with methylmercuric chloride and/or sodium pyruvate and allowed to remain for 6 hr in the dark. After the handling, the media were removed and the cells were washed with HBSS. After washing, 100 μL HBSS was added to each well and the fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Later on, the HBSS was replaced with DMEM containing 10% Alamar blue solution. The cell viability was measured at an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Susceptibility was calculated as ROS level/viability and is presented as a percentage of non-treated cells. Data stand for the mean ± S.D. (brackets) of six cultures. Significant differences were observed relative to the control grouping without pyruvate treatment. *p < 0.01 (E). Cell viability was measured using an Alamar blueish solution. The information correspond the mean ± S.D. (brackets) of three cultures. Significant differences were observed relative to the control group without pyruvate treatment. *p < 0.05, **p < 0.01.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This piece of work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article'south Creative Commons license, unless indicated otherwise in the credit line; if the fabric is not included under the Artistic Commons license, users will need to obtain permission from the license holder to reproduce the textile. To view a copy of this license, visit http://creativecommons.org/licenses/past/4.0/
Reprints and Permissions
Well-nigh this article
Cite this article
Lee, JY., Ishida, Y., Takahashi, T. et al. Transport of pyruvate into mitochondria is involved in methylmercury toxicity. Sci Rep six, 21528 (2016). https://doi.org/ten.1038/srep21528
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1038/srep21528
Further reading
Comments
By submitting a comment you agree to bide by our Terms and Customs Guidelines. If you lot discover something calumniating or that does not comply with our terms or guidelines delight flag it as inappropriate.
0 Response to "Can Pyruvate Travel Between Cytoplasm and Mitochondria"
Post a Comment